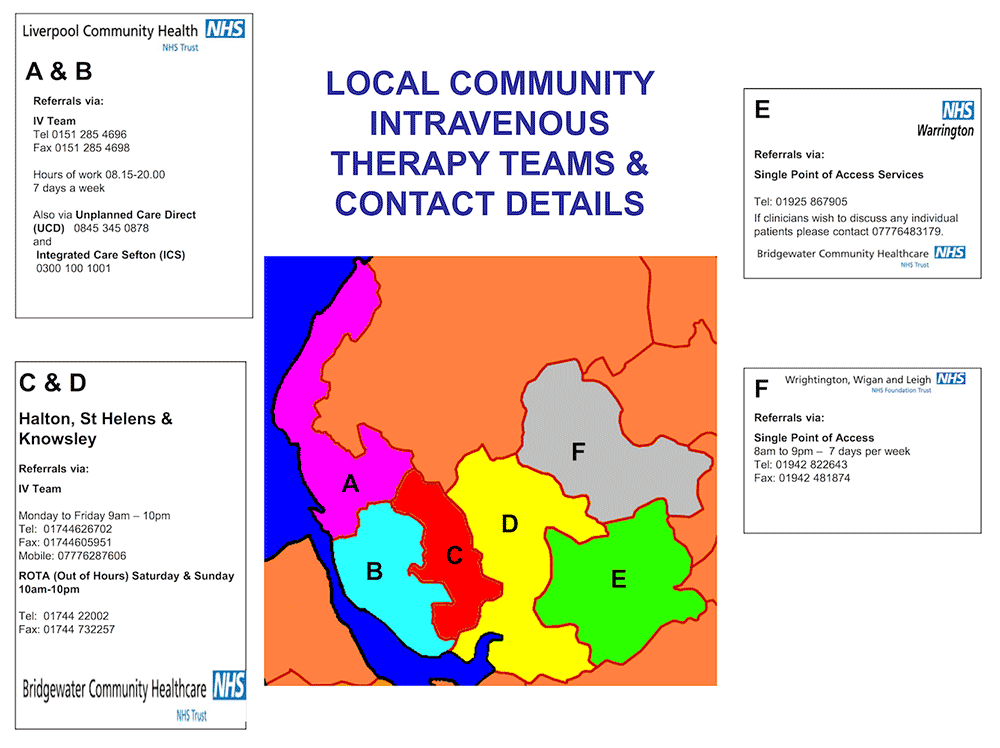
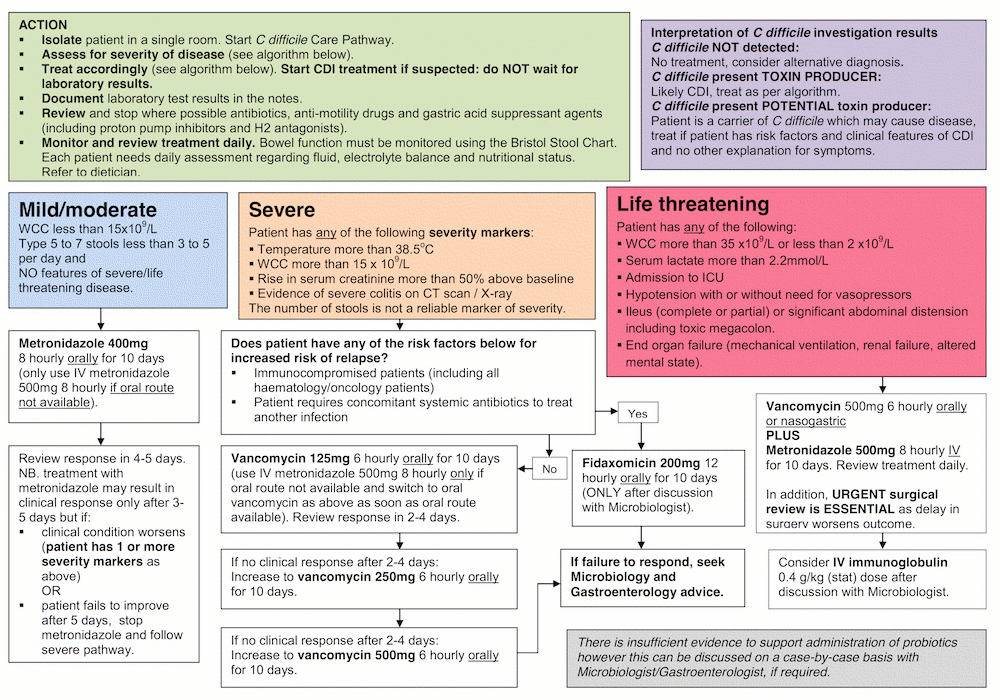
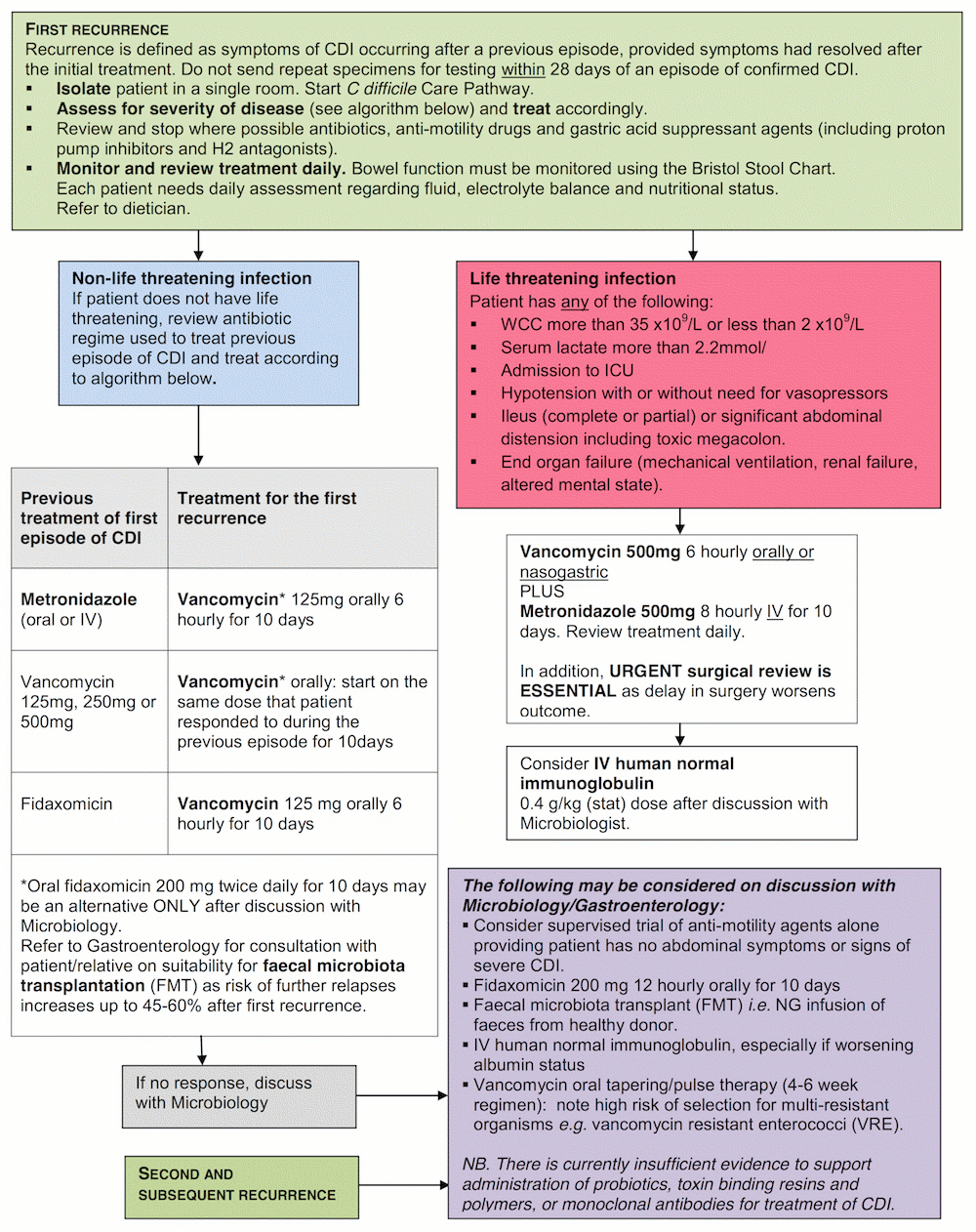
This is the disclaimer agreement for Mersey Micro.
Compliance with the Medical Devices Directive (93/42/EEC)
St Helens and Knowsley Teaching Hospitals NHS Trust confirms that the App is designed and manufactured to comply with the relevant Essential Requirements set out in the Medical Devices Directive (93/42/EEC). When used under the conditions and for the purposes intended, the App will not compromise the clinical condition or the safety of patients, or the safety and health of users or, where applicable, other persons.
No warranties
Subject to the paragraph above, the information within, and the operation of, the App is provided on an "as is" basis without any representations or warranties, express or implied, and St Helens and Knowsley Teaching Hospitals NHS Trust makes no representations or warranties in relation to it.
You acknowledge, agree, and understand that the App is for use by medical professionals only and that it is intended to be used as a tool and a guide to the prescribing of antibiotics to patients only. The App is designed with safety functions in order to eliminate or reduce risks as far as possible. However, nothing in the App should be considered to be a substitution for medical expertise, experience and judgement.
We will endeavour to keep the information on the App complete, accurate and/or up to date. However, due to the nature of the information being provided information contained in the App may quickly become out-of-date. Without prejudice to the generality of the above paragraph, St Helens and Knowsley Teaching Hospitals NHS Trust does NOT warrant that:
- the App, or the information on the App, will be available all of the time; or
- the operation of the App will be error-free; or
- the information on the App is complete, true, accurate and/or up-to-date.
Liability
Under no circumstances will St Helens and Knowsley Teaching Hospitals NHS Trust be liable to you or to any other person for any indirect, special, incidental, exemplary, consequential or other damages arising out of any use or misuse of the App, even if St Helens and Knowsley Teaching Hospitals NHS Trust is advised of the possibility of such damages being awarded.
Without prejudice to the generality of the above paragraph, St Helens and Knowsley Teaching Hospitals NHS Trust shall have no liability arising out of any use of the App which is not in accordance with the instructions for use and/or under the conditions and/or for the purposes intended, for:
- Any loss or injury caused, in whole or in part, by the procuring, compiling, or delivering of the App or the information in it;
- Any errors, omissions, or inaccuracies in the App or the information in it; or
- Any decision made or action taken or not taken in reliance on the App or the information in it.
Intellectual Property Rights
The App and the information within it are protected by copyright and other intellectual property laws in numerous countries around the world and St Helens and Knowsley Teaching Hospitals NHS Trust expressly asserts its ownership of and reserves all of its rights around the world in such intellectual property rights.
Usage and age restriction
The App is intended for use by persons aged eighteen years or older who are qualified medical professionals. Persons under the age of eighteen years must not access or use the App.
Indemnification
You agree to indemnify and hold St Helens and Knowsley Teaching Hospitals NHS Trust harmless from any claim or demand, including costs and expenses, made by any third party as a result of your use or misuse of the App which is not in accordance with the instructions for use and/or under the conditions and/or for the purposes intended and any action you take which is or may be based on the information contained within the App.
Governing law
This disclaimer and your use of the App and the information in it shall be governed by and construed in accordance with the laws of England and Wales and any dispute will be submitted to the English courts.
Modification
St Helens and Knowsley Teaching Hospitals NHS Trust may, at its sole discretion, modify this disclaimer and the App and the information within it in whole or in part at any time for any reason without giving notice to you.
You must check the disclaimer page of the App prior to each use of the App, whether prior or otherwise. Such modified terms and conditions shall supersede these terms and conditions and shall become binding when published online on the Site.
The app can be updated to all users over a short time period assuming network coverage for the users portable device, so any problems can be quickly corrected.